Table of Contents
INTRODUCTION
Patients with chronic pain have sometimes been recommended to use opioids to relieve pain but in certain cases like those suffering from Chronic Obstructive Pulmonary Disease (COPD), respiratory distress is always common. As such, opioids use increases the risks or susceptibility to respiratory complications as an implication for the need to find out alternatives to chronic pain management (Niesters, Overdyk, Smith, Aarts, and Dahan, 2013). More so, COPD patients are at higher risks of developing respiratory distress because the disease itself entails breathing problems or difficulties thereby compelling the need to find alternatives (Tas, Mıstanoglu, Darcın & Kececioglu, 2014). Therefore, for this study, the aim will be to find alternative medicine to managing chronic pain in COPD patients, with the emphasis on selecting the medication with the least respiratory distress.
Particularly, there is a considerable body of research currently focusing on identifying or outlining some of the respiratory implications of using tramadol to control and manage chronic pain. In this regard, existing evidence suggests that tramadol is considered safer than other opioids due to its respiratory effects as an implication that the medication is the best fit for COPD patients to control chronic pain (Tas, Mıstanoglu, Darcın &nKececioglu, 2014). On the other hand, there is substantive evidence indicating there have been rare cases of respiratory depression cases associated with tramadol in the clinical practice (Mahesh & Kaparti, 2014). Conversely, chronic pain can be managed through medications like fentanyl topical, a drug common with opioid-tolerant patients working by binding opioid receptors and in return, blocking the pain receptors to the brain (Bedirli, Egritas, Cosarcan & Bozkirli, 2012). However, fentanyl has been associated or linked with certain side effects like anxiety that causes more respiratory desperation as an implication that the drug may not be suitable for COPD patients (Tas, Mıstanoglu, Darcın & Kececioglu, 2014). Therefore, this study will seek to confirm that tramadol causes less respiratory distress among COPD patients in comparison to fentanyl topical, through a mixed research design or triangulation (experimental design and qualitative interviews). The patients will be exposed to drug treatments, and the effects monitored or recorded, then the hypothesis tested through a questionnaire.
Rationale
The rationale of this research is to clearly state the differences in using tramadol and fentanyl topical among COPD patients experiencing chronic pain. For this case, the focus will be to present findings ascertaining that tramadol presents less respiratory distress in comparison to fentanyl topical. On the other hand, the research is set to build on the existing knowledge confirming the positive effects of tramadol on respiratory rates since the drug does not affect respiratory stability (Tas, Mıstanoglu, Darcın & Kececioglu, 2014).
Some of the major assumptions that will be held in this study will focus on the effectiveness of tramadol in treating chronic pain. For one, a major assumption that the proposal will be based on is that tramadol is an effective and useful medication with limited side effects and improves the quality of life of patients (Tas, Mıstanoglu, Darcın & Kececioglu, 2014). Another assumption is that fentanyl topical does not apply with chronic pain COPD patients given the implications of anxiety that leads to increased or more respiratory distress (Mahesh & Kaparti, 2014).
For the experimental treatments or design, the hypothesis will be: Tramadol causes less respiratory depression among COPD experiencing chronic than topical fentanyl.

However, to ascertain the research findings, from the experimental designs, the patients will be interviewed, through a qualitative design and this will lead to probing the following questions.
The research objectives
- To find out if tramadol reduce or cause less respiratory depression in COPD patients experiencing chronic pain
- To determine if topical fentanyl cause more or less respiratory depression among COPD patients experiencing chronic pain
- To examine if tramadol increase or reduce CO2 and 02 exchange tensions among COPD patients experiencing chronic pain
- To examine fentanyl topical increase or reduce CO2 and 02 exchange tensions among COPD patients experiencing chronic pain
Theoretical framework
The theoretical framework below concerns or focuses on the effects of medical intervention on the variables including respiratory distress(breathing rate), CO2 and 02 exchange tension, and the efficacy in pain relief among COPD patients. The framework will guide or be used to measure the outcomes for the intervention methods and as such, compare the results both from the experimental treatments and the qualitative interviews.
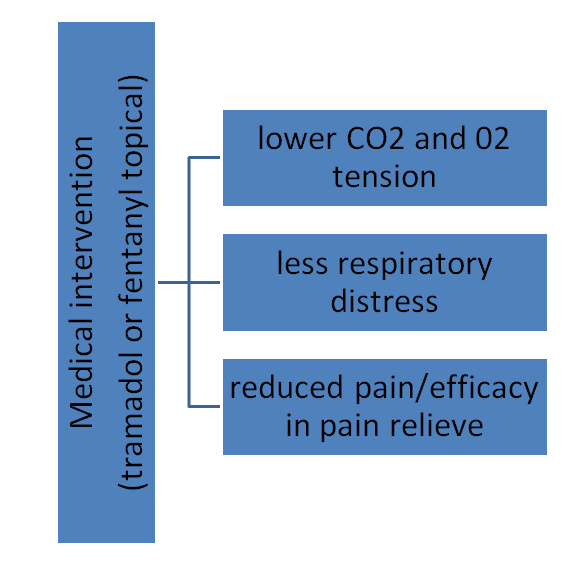
Existing researchers contend that no respiratory depression has been associated with tramadol in clinical practice (Mahesh and Kaparti, 2014). On the other hand, previous studies indicate that with the use of tramadol, patients do not indicate any altered-tidal CO2 changes and in other words, their respiratory functions as compared to the use of other medication like pethidine or morphine (Bedirli, Egritas, Cosarcan & Bozkirli, 2012). Nonetheless, empirical studies have indicated that respiratory rates, or the CO2 and arterial tensions, are reported to be normal among breathing patients who undergo surgeries with the prescription of epidural tramadol in chronic pain management (Tas, Mıstanoglu, Darcın & Kececioglus, 2014). On the other hand, some studies refute the suitability of tramadol in managing chronic pain citing the possibility of respiratory depression, especially those that observed respiratory depression emanating from dose-dependency after tramadol high doses in cats, with the indication of similar results on humans (Mahesh & Kaparti, 2014). However, the evidence on overdose-respiratory depression associated with tramadol is refutable because such would be subject to exceeding the quantity of medication thereby supporting that tramadol presents less respiratory depression. In contrast, fentanyl topical is restricted to use in chronic pain management among COPD patients because the drug can only be used in specific cases (Mahesh & Kaparti, 2014). For instance, other studies suggest that fentanyl topical only assists with acute pain management and as such, is restricted to patients’ requirement of the medication for a short period Bedirli, Egrets, Cosarcan & Bozkirli, 2012). Moreover, substantive evidence confirms that fentanyl drugs are not suitable for patients who have serious respiratory compromise like COPD because of the side effects like anxiety that can increase respiratory distress among patients (Mahesh & Kaparti, 2014).
LITERATURE REVIEW
Conversely, there has been a considerate body of research seeking to explore or find out some of the reasons as to why tramadol is the preferred analgesia with findings indicating that tramadol improves patient outcomes. Besides, explore that the use of tramadol in treating chronic pain has been studied with findings confirming the usefulness of the drug or the medication in relieving pain (Russell, von Ungern-Sternberg & Schug, 2013). Moreover, a study compared the effectiveness of tramadol with fentanyl in Dilation and Curettage among expectant women, and the findings indicated that tramadol had better or best analgesia effects as well as better safety outcomes in comparison to fentanyl (Tas, Mıstanoglu, Darcın & Kececioglus, 2014). Therefore, from the findings, the researchers concluded that tramadol has better analgesic efficacy than fentanyl, and may be considered or preferred because of its better respiratory outcomes because the medication presents better respiratory stability in patients who have undergone surgery. Nonetheless, tramadol and fentanyl have been compared among pediatric patients who were undergoing propofol-based sedation (Bedirli, Egritas, Cosarcan & Bozkirli, 2012). The results indicated that fentanyl significantly reduced the saturation during the 5th and 10th minutes of sedation but in comparison, the findings indicated that the adverse effects, as well as recovery time, were found to be significantly lower in those dosed with tramadol in comparison to the fentanyl treatments. In this case, the research findings confirm that tramadol results in less respiratory depression in comparison to fentanyl topical (Bedirli, Egritas, Cosarcan & Bozkirli, 2012).
From the existing literature findings or report, it is clear that tramadol is effective analgesia, effective in reducing pain due to its high affinity to pain receptors while at the same time maintaining respiratory stability in comparison to fentanyl topical that has been shown to lead or result in higher levels of anxiety that can cause respiratory depression. In this case, the existing knowledge or information will ground this research seeking to confirm the effectiveness of tramadol in resulting in lowering respiratory distress among COPD patients than fentanyl topical.
THE METHODOLOGY
The study will both a quantitative approach in testing the hypothesis and answering the research question. A quantitative design will be used for the experimental treatments or the doses given to the patients since it is the suitable design for measuring values from changes in the breathing rate, CO2 and O2. A questionnaire will be used as a data collection tool since it is practical, more poised to provide larger extensive information, easy quantification of the results, and as such, analysis can be carried out more scientifically (Bekhet & Zauszniewski, 2012).
Experimental treatments (quantitative design)
For the experimental treatments, the sample size to be used in this case is going to be 8, and this will be divided equally for tramadol and fentanyl topical treatment or tests. Notably, 4 patients will be used for tramadol, and another 4 used on fentanyl topical. As such, random sampling will be utilized in this case because the sampling criteria are free from biases and lead to equal representation or selection of each subject within the study population. The experimental design will imply the use of a quantitative model because a statistical analysis will be used in exploring the p-Values of the relationship between the input of the independent variable and the output variables. A quantitative design is appropriate because it will help with the determination of the statistical values to prove the statistical significance of the research (Bekhet & Zauszniewski, 2012). Moreover, the study is set to select a sample size that will lead to a positive statistical significance to determine or improve the reliability of the research. In this case, with the reduced bias, the sampling method is set to provide the chance or opportunity for carrying out randomized control trials and getting the same results.
The inclusion criteria for the study subject will revolve around COPD diagnosis and chronic pain. Therefore, only individuals with COPD while at the same time suffering from chronic pain will be included. Care will also be taken not to include patients currently under other opioid medication as this may compromise the findings. In this case, freshly diagnosed cases of chronic pain, 2 weeks old will be included in the sample design.
For the study, the elements of a research approach will be used, whereby the independent variable (medical intervention, either tramadol or fentanyl topical), as the input, with be measured against the outputs of respiratory rates or the breathing levels, response to pain, and the CO2 exchange among the study subjects or the patients.
We can do it today.
Data collection
The data will be collected by nurses, since they are the ones with the records of the patients who have been given the chronic pain management medication, as an implication that the setting of the study will occur within a health care facility or hospital context. The data will be collected after the first dose is over for each pain management medication whereby the nurses will measure record the observable changes in the breathing rate, respiratory distress (CO2 and 02 exchange tensions), and overall response to pain and indicate them in the questionnaires.
The research also has incentives for protecting human subjects, and this will involve the provision of a consent form that highlights the voluntary nature of the research. In this case, the consent form will indicate that participation in the research is voluntary, with no potential harm to those who choose not to participate, and that the information will be used upon individual consent. On the other hand, confidentiality will be maintained to the levels that the researcher can control, but all participants will be assured of anonymity when presenting the results of the findings.
Data Analysis
For the experimental design, or the quantitative design, a statistical analysis will be used, whereby a regression analysis will be used to determine the p-values from the relationship between independent variables (the drug treatments or tramadol and fentanyl topical) against the independent variables (CO2 and 02 tension, breathing rate). The independent variables to consider will include ‘CO2 and 02 exchange or tension rates’, ‘the rates of breathing, response to pain’, finally, ‘noted respiratory distress’. The proposal will present or organize the data in a tabular form thereby comparing and contrasting the statistical findings from tramadol treatments with the fentanyl topical treatments against the benchmarked patient outcome.
Implications for the nursing profession
The subsequent study is set to contribute to the nursing knowledge by providing an evidence-based criterion for managing chronic pain among COPD patients and taking control of their respiratory distress. In this case, the findings are set to support alternatively safer medication like Tramadol that induces less respiratory depression in comparison to another analgesia like fentanyl topical.
The research, with the identification of proper and appropriate sample size, is set to provide a higher statistical significance and p-value as an implication that the results of the findings will not occur by chance by rather an indication of the positive relationship between the independent and the dependent variables. In this case, with reliable statistical significance and the p-values, this research will possibly be replicated in the same research.
The findings are set to benefit some of the areas like chronic pain management among COPD patients, alternatives to opioid use in chronic pain management and the recommended best pain management medication with higher efficacy and resulting in respiratory stability.
- Bedirli, N., Egritas, O., Cosarcan, K., & Bozkirli, F. (2012). A comparison of fentanyl with tramadol during propofol-based deep sedation for pediatric upper endoscopy. Pediatric Anesthesia, 22(2), 150-155. Doi:10.1111/j.1460-9592.2011.03707.x.
- Bekhet, A. K., & Zauszniewski, J. A. (2012). Methodological triangulation: an approach to understanding data. Nurse Researcher, 20(2), 40-43. Doi.org/10.7748/nr2012.11.20.2.40.c9442.
- Mahesh, T., & Kaparti, L. (2014). A randomized trial comparing efficacy, onset, and duration of action of pethidine and tramadol in the abolition of shivering in the intraoperative period. Journal of clinical and diagnostic research: JCDR, 8(11), GC07. Doi:10.7860/JCDR/2014/10584.5148.
- Niesters, M., Overdyk, F., Smith, T., Aarts, L., & Dahan, A. (2013). Opioid-induced respiratory depression in pediatrics: a review of case reports. British journal of anesthesia, 110(2), 175-182. Doi: 10.1093/bja/aes447.
- Russell, P., von Ungern-Sternberg, B. S., & Schug, S. A. (2013). Perioperative analgesia in pediatric surgery. Current Opinion in Anesthesiology, 26(4), 420-427. Doi: 10.1097/ACO.0b013e3283625cc8.
- Tas, A., Mıstanoglu, V., Darcın, S., & Kececioglu, M. (2014). Tramadol versus fentanyl during propofol-based deep sedation for uterine dilatation and curettage: A prospective study. Journal Of Obstetrics & Gynaecology Research, 40(3), 749-753. Doi:10.1111/jog.12259
