Table of Contents
Important points noted in the research
The important points as discussed in this research are the production of a plicamine-type indoloisoquinolines molecules through the use of simple materials and a four-phase reaction. The main product from which plicamine molecules were to be derived from was imine. The research has also reported the increasing demand and need to synthesize reagents using components that are compatible to the environment. Furthermore, the paper has outlined the characteristics of good synthetic designs concluding that good synthetic designs should give access to particular target molecules and to a library of analogs that closely resemble one another. Following the aforementioned qualification, the authors have noted that the suitable reactions are multicomponent ones for instance the Ugi four-component reaction (Ugi-4CR). It has also been established that synthesis of complex compounds require complex reagents.
The authors have also highlighted the type of alkaloids that are suitable in the synthesis process of complex compounds and outlined the reasons why these alkaloids are preferred. Specifically, the research has focused on alkaloids belonging to the Amaryllidaceae family through the use of Ugi-4CR process. Any process has optimum requirements that enable it to work efficiently and effectively. The research has also outlined that it is through challenges that one can be able to come up with something new, something that can solve existing problems. In the end, the researchers came up with a waste-minimization approach for producing plicamine-type molecules with complex structures. Finally, the researchers have outlined different approaches that were used to test the effectiveness of their proposed multicomponent reaction process and this has been presented in different schemes, from scheme 1 – 5.
The sequence of the reactions described in the research
The research outlines different reactions in five different phases, scheme 1 – 5 as discussed in the subsequent paragraphs. In scheme 1, two reaction processes were used. First, the Ugi-4CR consisting of four components, p-hydroxybenzaldehyde, piperonyl amine, a carboxylic acid and an isocyanide, was used in the synthesis of a bisamide (Mijangos & Miranda, 2016). This process, however, will result into lack of -lactam that is usually present in placamines. The intramolecular Michael addition reaction is usually used in this phase to synthesize -lactam. Different components of the Ugi-4CR components were chosen and optimized under different conditions. For instance, in the first reaction of scheme 1 of Ugi-4CR, t-butyl Isocyanide and p-methoxyphenylacetic acid worked perfectly under 600C temperature that resulted from microwave heating (Mijangos & Miranda, 2016). The mixture above resulted to the preformation of MeOH.
In the schema 2, a coupling process referred to as the intramolecular oxidative de-aromatization-phenolic process was investigated. In order to investigate this process, all the Ugi-4CR components were mixed with PIFA in a solution of 2, 2, 2-trifluoroethanol and the mixture was maintained at a temperature of –250C (Mijangos & Miranda, 2016). This reaction compelled phenolic bisamide to crystalize leading to the production of spirodionone. The formed product, spirodionone, is very unstable in acidic environment and for this reason, the reaction of this phase climaxes with the separation of spirodionone.
In scheme 3, the reaction was controlled by adding different equivalents of DBU to the mixture produced in scheme 2 above. The first reaction was the endeavor to synthesize indoloisoquinolindione by adding intramolecular aza-Michael but the reaction failed owing to the presence of acidic conditions in the mixture. 1 equivalent of DBU in acetonitrile had to be added to this mixture in order to facilitate the cyclization of phenolic bisamide. The above mentioned was a two-phase reaction which was not economical as far as chemicals were concerned and the researchers opted for an economical reaction dubbed “one-pot” reaction. With the “one-pot” reaction, however, even 7 equivalents of DBU at room temperature could not result into cyclization of phenolic bisamide (Mijangos & Miranda, 2016). The final reaction in this phase involved removal of the acidic solvent through the oxidative phenol coupling process and this facilitated cyclization of phenolic bisamide upon addition of 4 equivalents of DBU in acetonitrile.

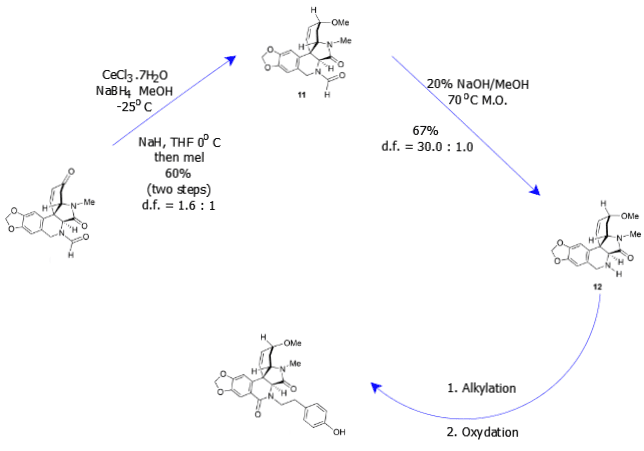
While scheme 1, 2 and 3 focused on the necessary environment for the synthesis of plicamine-type molecules, that is, suitable temperature and the appropriate basicity or acidity of the solution, scheme 4 focused on altering the different components of Ugi-4CR with the aim of investigating their effects on production of plicamine molecules. This phase involved checking the structure of the molecules produced by investigating the structure of the compounds formed through X-ray crystallography (Mijangos & Miranda, 2016). Some of the components that were used in this study include t-butyl, benzyl, cyclohexyl, and isopropyl and methyl isocyanide.
The fifth phase, scheme 5, involved the production of indoloisoquinolines using all the necessary conditions that were established in the preliminaries studies 1 – 4. Scheme 5 involved the synthesis of seventeen plicamine molecules by altering the components of the Ugi-4CR as investigated in scheme 4. This reaction phase involved the use of p-Meo-phenylacetic, acetic, valeric, 3-cyclophenyl propionic, and formic acid as the main components of the Ugi-4CR (Mijangos & Miranda, 2016). Appropriate combination of the above four components lead to the synthesis of tetracyles.
What I think about the difficulties and challenges in the research conducted
This researchers faced a lot of challenges as they lacked adequate information from reviewed literature. According to me, however, it is through the challenges that the researchers were able to come up with simple and waste-minimization approach of synthesizing plicamine-type molecules. Taking a look at the first four scheme phases, the researchers had to test different Ugi-4CR components and reaction environments that would be appropriate in the production of plicamine-type molecules. It took four phases of trial and error to come up with an effective and workable method. This clearly demonstrates that challenges brings a breakthrough. On the other hand, however, I have learnt that challenges leads to wastages of resources. In the event that one lacks information in relation to a particular subject matter, they will use a lot of resources in the process of trying to understand it. Each of the four phases involve altering the Ugi-4CR components and different parameters in order to come up with the appropriate outcome. For this reason, a lot of time was utilized in the process of finding the optimum conditions required in the synthesis of plicamine molecules. In general, however, challenges are there to enable one discover and achieve things that others were unable to.
Any other interesting point noticed
While most of the chemical reactions usually require raised temperatures, due to the existence of bonds in them, in order to change from one form to another, it was surprising to realize that one of the reactions required very low temperature of up to –250C to take place. I also noted that complex compounds could only be synthesized from complex molecules. For instance, indolo[3, 3a–c]isoquinolione could only be synthesized complex alkaloid compounds. It is, therefore, the reacting component that determines the nature of the compound to be synthesized and not necessarily the complexity of the process. Finally, I have learnt that in an exploratory research, one should have an open mind so as not to limit the process required to attain a particular result. In this research, the researchers had failed to archive the desired product 9a by cyclizing 10a to 9a through and addition process but it was easier to archive the same outcome by a reduction process (phenolic coupling process) which led to the conversion of 8a to 9a. If the researchers had dwelt in trying to covert 10a to 9a, it would have taken them a lot of time and resources. They were open to any other process that would give them the desired results.

Other latest research related to this one
Multicomponent reactions have become the most preferred reactions when it comes to the synthesis of organic compounds. This is due to their ability to produced complex molecules with diverse characteristics from simple compounds and their reactions usually takes a short period of time. Since 1959, Ugi-4CR has undergone a couple of modifications to make it more effective. A couple of variants had to be used in this research to produce the required plicamine-type molecules. These variations and modifications have in time resulted to the emergence of (Ugi-5C-4CR) which has more reacting functional groups and has a high ability in the synthesis of organic compared to Ugi-4CR (Dawidowski et al., 2014). More research on Ugi-5C-4CR is still in progress. It is hoped that the breakthrough in these researches will aid in the expansion of molecular diversity.
Reactions and their plausible detailed mechanism
The first reaction, as depicted in scheme 2, involved the preformation of imine in MeOH. Chemically, the reaction involved is expressed below:
p-hydroxybenzaldehyde+piperonyl imine+t-butyl isocyanide+p-methoxyphenylacetic acid →imine
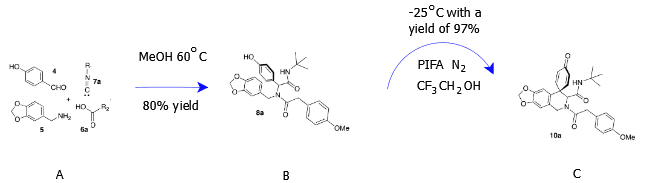
The chemistry represented by the curly arrow above is oxidative chemistry. The Ugi adduct (B) is oxidized to a spirodionone (C) through a coupling process. Taking a close look at the compound C, it has more oxygen atoms compared to compound B indicating that oxidation has taken place.
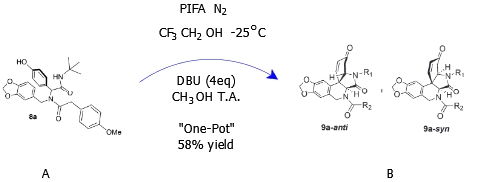
Depicted by the above reaction, is combination chemistry in which spirodionone has combined with PIFA with the addition of 4 eq. DBU to produce plicamines (B). Nevertheless, the plicamines produced in B were in a mixture of diastereomers and inseparable. The final reaction is oxidative in nature as diastereomer is converted into final plicamine-type molecule through oxidation.
- Dawidowski, M., Sobczak, S., Wilczek, M., Kulesza, A., & Turło, J. (2014). Expanding the substrate scope of ugi five-center, four-component reaction U-5C-4CR): ketones as coupling partners for secondary amino acids. Molecular diversity, 18(1), 61-77.
- Mijangos, M. V., & Miranda, L. D. (2016). Multicomponent access to indolo [3, 3a-c] isoquinolin-3, 6-diones: formal synthesis of (±)-plicamine. Organic & biomolecular chemistry, 14(15), 3677-3680.

