Table of Contents
Introduction
Over the years, the global demand for renewable energy has increased tremendously. The increasing levels of global warming has led to pressure for countries to look for renewable energy sources with the aim of achieving sustainable development. With various environmental declarations having been signed but no results, there is need for long-term solutions that will reduce the amount of greenhouse gases that are being released into the atmosphere. Borole (2015) defines microbial fuel cells (MFCs) as, “electrochemical devices that use microbes as catalysts instead of inorganic catalysts to drive the anodic and/or cathodic reactions to produce electricity… microbial electrolysis cells (MECs) as a different manifestation of BES that generate hydrogen using less than half the voltage or electrical energy needed for conventional water electrolysis” (Borole, 2015, p. 55). Research has proved that both Microbial Fuel Cells (MFCs) and microbial electrolysis cells (MECs) can be used to produce renewable hydrogen through energy and economically feasible means (Borole, 2015). These innovations offer a breakthrough to some of the challenges that the world is facing in regards to the demand for renewable energy. The paper will discuss how the production of clean energy (renewable hydrogen) could be achieved by combining the principles of MFCs and MECs. The possibilities of microbial fuel cells as a potential unconventional energy source in the near future and an analysis of the different performance studies on microbial fuel cells will also be provided.
Body/Discussion
Challenges in Fuel Yield
There has been a global consensus in utilizing non-conventional sources of energy for generating electrical and thermal power. Such initiatives are mandated to preserve fossil fuels and for reducing the amount of greenhouse emissions (GHE). The field of energy and low carbon technologies has witnessed radical transitions during the past decade (Rahimnejad et al., 2015). The major focus of such transitions is to enhance the production of renewable sources of energy. Based on such philosophy there has been a drastic increase in the usage of tidal, wind, and geothermal energy across the globe. However, the possibility of fuel yield from such sources is not only limited but ineffective too. Hence, focus has been shifted on other sources that could act as viable fuel sources. One such innovation is the development of fuel cells such as proton exchange fuel cells, solid oxide fuel cells, molten carbonate fuel cells, alkaline fuel cells, and microbial fuel cells. The energy efficiency of these fuel cells has been a matter of long debate. However, microbial fuel cells have shown promising results as an alternative energy source to overcome crisis in the power sector.
Overview of Microbial Fuel Cells (MFCs)
MFCs use specific groups of microbes based on their ability to donate electrons superficially through the electron transport chain. Hence, a modified MFC can achieve effective electrohydrogenesis or bio-catalysis. The process strictly operates under anaerobic conditions and the hydrogen generated through this process is estimated to be 8 to 9mol/mol of glucose or 2.9mol/mol of acetate present as the organic substrates (Logan et al., 2006). The efficiency of hydrogen production is estimated to be more cost-effective than chemical purification and extraction of hydrogen. The electrochemical operation of modified MFCs (such as MECs) is almost identical to the MFCs with the exception of use of external power source. The external power source is required for reducing the protons to hydrogen at the cathode. The hydrogen collected at the cathode is compressed and made into hydrogen fuel cells. Energy harnessed can therefore, be used to run the economy.
Electrolysis of water generates oxygen and hydrogen gas. The reaction has a standard electric potential of -1.23 volts, which reflects that a potential difference of 1.23 volts is required to split water into the respective gaseous components. Based on the same principle hydrogen fuel and breathable oxygen can be prepared (Abbasi and Abbasi, 2011). First, a direct current (DC) supply is connected to both the electrodes of the MFC. These electrodes are made up of inert materials (such as platinum or stainless steel) and are positioned in the water. Hydrogen appears at the cathode where electrons enter the water, while oxygen gas is liberated at the anode. Considering the faradic efficiency to be ideal, the amount of hydrogen that is generated can be assumed to be twice than that of oxygen produced (Abbasi and Abbasi, 2011). Moreover, the generation of both these gases can be assumed proportional to the total electric charge conducted through the solution. However, the faradic efficiency is jeopardized in most MFCs and the yield of hydrogen or oxygen is not ideal as predicted. This is because in most of such cells opposing side reactions take place to reduce their yield
Recently, Microbial Fuel Cells (MFCs) have generated a lot of interest for its possibilities as a potential fuel resource. MFCs have the ability to convert organic matter into electricity in presence of diverse microbial populations. Different studies have highlighted the beneficial effects of MFCs on domestic and industrial wastewater treatment (Lalaurette et al., 2009). These cells have proved their potential in the Activated sludge process (ASP). However, their role as a potential source of fuel is either inconclusive or underreported. The global demand for conventional and unconventional sources of energy is always on the rise. Presently, most of the global energy demands are met by conventional sources of energy such as fossil fuels. However, the depletion of conventional sources of energy has shifted the focus of concerned stakeholders towards different forms of non-conventional, renewable, and alternative sources of energy.

One such renewable form of non-conventional energy is fuel cells. Microbial fuel cells are a special type of fuel cells that have dual advantage; microbes that are added into organic matter are converted into electricity and purification of wastewater. Hence, microbial fuel cells could offset the operating costs of wastewater treatment plants. Any MFC is usually comprised of two chambers that include the anode chamber and the cathode chamber. The organic material is oxidized in the anode chamber by the microbes. The electrons that are generated in such process are transferred to the anode either by an artificial electron transporter that is introduced into the anode chamber or directly from the bacterial electron transport chain itself. Once the electrons are transported to the anode, the external circuits take them to the cathode the terminal electron acceptors eat up the electrons. The operation of a typical MFC is shown in fig 1 (Logan et al., 2006, Borole, 2015).
Principle of Operation of MFCs and MECs
MFCs can operate with wide variety of substrates (or fuels) such as glucose, acetate, other monosaccharide, complex carbohydrates (starch) and biodegradable organic matter present in food, swine, and domestic wastewaters. However, the amount of electricity produced by the MFCs depends on the type of microbial substrate (Lalaurette et al., 2009). Although MFCs were initially used for treating wastewater, MFCs are increasingly used as bioelectrochemical systems (BES) in the form of microbial electrolyzers (MEs) and microbial electrolysis cells (MECs). MEs and MEC are different variants of BES systems that have the potential to generate hydrogen by using less than half the voltage or electric energy that is needed for the conventional electrolysis of water. The lower requirement of electric energy is supplemented by chemical energy (Sleutels et al., 2012). The chemical energy is derived either from organic or from the reduced inorganic substrates because of microbial catalysis on them. The electrons are derived from the substrates and are converted to hydrogen at the cathode of the MFCs. The entire process is accomplished under room temperature. The catalysts present at the cathode can be either metal-based or microbe-based. Hence, MFCs have the ability to produce not only electricity and hydrogen, but is equally versatile in producing bio fuels, chemicals, and bio products. The feasibility of hydrogen production and other functional attributes of MFCs depend on the synergy between electro catalysis and bio catalysis (Lalaurette et al., 2009).
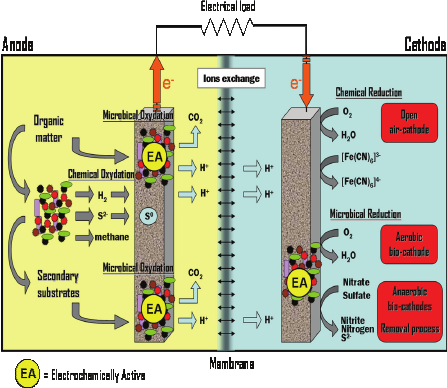
Fig. 1. Operation of typical MFCs. From ‘Microbial fuel cells—an option for wastewater treatment’ (Duteanu et al., 2010, p. 1070).
Synergy between electro catalysis and biocatalysis in MFCs
MFCs are well known for their efficiency in producing electricity and hydrogen. The higher efficiency of MFCs could be attributed to the molecular dynamics of electro catalytic reactions. Compared to thermo catalysis, electro catalysis is considered more efficient. In thermo catalysis, vibrational energy is used to increase the rate of the reaction, while in electro catalysis voltage fluctuations is the driving force for conducting electro catalysis. Electro catalysis has certain advantages over thermo catalysis because it minimizes the loss of energy to the environment (Gorby et al., 2006). Hence, the conversion efficiency of electro catalysis is higher than thermo catalysis. On the other hand, bio catalysis is a type of low-temperature catalytic activity whereby it works by lowering the activation energy of the reacting substrates. Combining the principles of electro catalysis and bio catalysis, the efficiency of BES systems are significantly improved. BES systems ensure the continuity of electron flow from the substrate to the product. Hence, in BES systems, the electrons first flow through the bio catalytic reactions, which are then followed by electro catalytic reactions. Such systems ensure continuous path within the electrical circuit. Such systems have been complemented further with the discovery of nanowires that helps to communicate between biological systems and electrodes.
Biological nanowires efficiently transfer electrons between inorganic substrates and organic entities. For example, Pili-based nanowires have been identified in different bacterial species such as G. sulfurreducens and S.oneidensis. These nanowires are capable of transferring electrons from the microbes to the anode of MFCs. Borole (2011) states, “direct interfacing of electron producers and electron sinks has translated into high efficiency in MFCs.” Likewise, MECs have also exhibited high efficiency in the generation of hydrogen from acetate. The cuolombic efficiency of hydrogen generation from acetate is estimated to be greater than 90% and the overall efficiency is rated to be approximately 82% (Gorby et al., 2006).
We can do it today.
Economic and energy efficiency of MFCs/MEC
Zero-energy wastewater treatment is one of the major visions of environmentalists and environmental scientists. Such initiatives are targeted to address the scarcity of water and BES systems hold the promise of bringing the vision into reality. On the other hand, scientists are also in search of renewable sources of hydrogen and therefore, they have explored the genesis of renewable hydrogen from different sources of non-conventional energy such as solar, hydal, wind, and biomass (Borole, 2011). However, commercial production of renewable hydrogen from these sources is far from reality. MECs have exhibited the efficiency and ability to produce renewable hydrogen in a commercial scale from biomass and wastes. MECs can produce hydrogen from wastes and products of biomass hydrolysis such as sugars and organic acids. MECs can also convert lignin-degradation products, phenols and phenol-like compounds, and furan aldehydes into hydrogen at the bio anodes.
The economic considerations of MFCs are estimated in three ways that include production of hydrogen/electricity, potency of wastewater treatment, and production of clean and potable water. Different studies suggest that the economic feasibility of MFCs could be achieved with a current density of 25A/m2. Such feasibility can be achieved by reducing the internal electric resistance of the MFCs below 40 milliohm (Cheng and Logan, 2007). Such findings implicate that production of only electricity from MFCs might not be sufficient in explaining its operational feasibility. However, value additions such as potency of wastewater treatment and production of clean and potable water increases the economic feasibility of MFCs. On the contrary, the economic feasibility of MECs is much higher and visible compared to MFCs. MECs produce renewable hydrogen, which is valued higher than electricity generation (Abbasi and Abbasi, 2011). MECs hold the promise of being a cost-effective alternative for producing renewable hydrogen compared to other sources. It is estimated that MECs can bring down the cost of production of renewable hydrogen 2. U.S. Dollars/ kg. The specific cost reduction goals of such renewable hydrogen production are focused to reduce cathode costs to 50 U.S. Dollars/square meter with a target rate of commercial hydrogen production to 4 litters/l reactor a day. The current density that is required to ensure the economic feasibility of MECs is estimated to be 20A/m2. Such findings indicate that the electric efficiency of MECs is higher than MFCs (Cheng and Logan, 2007).
Commercial and renewable hydrogen production from MFCs/MECs
Any MFC consist of a cathode compartment and an anode compartment, which are linked by a salt bridge. The cathode and the anode are connected by external wires. The microorganisms present in the anodal compartment oxidize the organic matter (either introduced artificially or as a component of wastewater) to transfer the generated electrons at the anode Logan et al, 2006). The hydrogen ions are produced by the oxidation of half-reaction and pass through the membrane to the cathode. In the cathodal compartment, these hydrogen ions reduce oxygen to water. The resultant effect of both these reaction creates a potential difference, which aids in current flow.
The preparation of modified MFCs requires the assemblage of cathode and anode. The copper wires that make these electrodes are prepared. Holes are drilled in the plastic container through which the electrodes are introduced. The holes are also used to insert the air pumps in the cell. The air pump is placed at the cathode only (Borole, 2015). Next, a stainless steel mesh is cut into four pieces and attached to the respective electrodes. The salt bridge is next prepared by heating water, 30 grams of agar, and salt. The solution is left for solidification overnight. The conductive solution is inserted into the cathodal chamber, while benthic mud is placed in the anodal chamber. The electrodes are connected by multimeter to estimate the flow of electric current through the circuit. The hydrogen generation from the benthic mud depends on two vital steps that comprise electrolysis of water and electrolytic separation.
On the other hand, electrolytic separators produce hydrogen and oxygen from water and electricity. These appliances send controlled amounts of direct current into a fluid to break the bonds of the atoms that are within such fluid. In electrolytic separators, the glass tubes are connected by U-shaped plumbing fittings. Next, the stainless steel plates are cut in appropriate dimensions and holes are drilled in them. The sheets are then connected with each other and this arrangement is placed in both the glass tubes (Kolker, 2005). The U-shaped arrangement is placed in a stable position and water is filled in the glass tube with a little quantity of KOH. The external power is provided to the electrolytic separators by the MFCs itself. As a result, the electrolysis of water initiates with the production of hydrogen from water. The hydrogen that is liberated is collected for future use. A process of commercial production of hydrogen through MECs is shown in Figure 2 below.
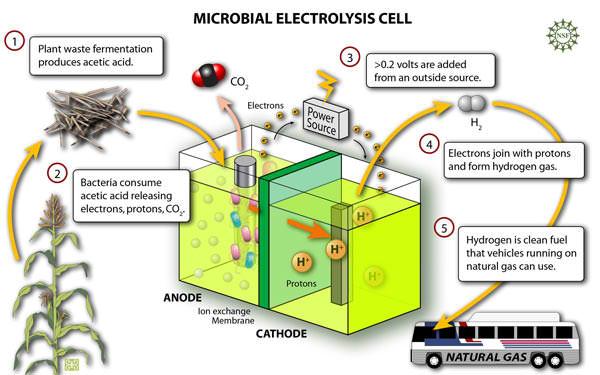
Fig. 2. Commercial production of hydrogen through MECs. From ‘Microbes Churn Out Hydrogen at Record Rate | NSF – National Science Foundation’ (Deretsky, 2007).
Conclusion
The aim of this paper was to discuss the use of MFCs and MECs in the production of renewable hydrogen. From the discussion, it is clear that with more resources and attention, the world can be able to increase the amount of hydrogen power. Modified MFCs and MECs cannot only generate renewable hydrogen and electricity but also holds the promise of being an energy efficient alternative for different water purification systems and reduction of waste. This means that both MFCs and MECs are advantageous because they have other beneficial purposes in producing sustainable and efficient systems making them economically feasible. Future research should be carried out different types of microbes that have superior efficiency in transferring electrons through their electron transport chain. Such initiatives could witness a faster production of hydrogen along with the total amount of hydrogen produced. Hydrogen produced through MECs could have a significant impact in overcoming fuel crisis in the near future. Therefore, heavy investment is required to achieve sustainable development. Government should work together with different stakeholders to ensure that these renewable energy sources are exploited optimally to reduce the amount of greenhouse gases being released into the atmosphere.
- Abbasi, T., and Abbasi, S., 2011. ‘Renewable’ hydrogen: Prospects and challenges. Renewable and Sustainable Energy Reviews, 15(6), 3034-3040.
- Borole, A. P., 2011. Improving energy efficiency and enabling water recycling in biorefineries using bioelectrochemical systems†. Biofuels, Bioproducts and Biorefining, 5(1), 28-36.
- Borole, A. P., 2015. Microbial Fuel Cells and Microbial Electrolyzers. Interface magazine, 24(3), 55-59.
- Cheng, S., and Logan, B. E., 2007. Sustainable and efficient biohydrogen production via electrohydrogenesis. Proceedings of the National Academy of Sciences, 104(47), 18871-18873. Deretsky, Z., 2007. Microbes Churn Out Hydrogen at Record Rate | NSF – National Science Foundation.
- Duteanu, N.M., Ghangrekar, M.M., Erable, B. and Scott, K., 2010. Microbial fuel cells—an option for wastewater treatment. Environmental Engineering and Management Journal, 9(8), pp.1069-1087.
- Gorby, Y. A., Yanina, S., McLean, J. S., Rosso, K. M., Moyles, D., Dohnalkova, A., and Fredrickson, J. K., 2006. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proceedings of the National Academy of Sciences, 103(30), 11358-11363.
- Kolker, E., 2005. Interdisciplinary Study of Shewanella Oneidensis MR-1’s Metabolism & Metal Reduction. Progress Report.
- Lalaurette, E., Thammannagowda, S., Mohagheghi, A., Maness, P., and Logan, B. E., 2009. Hydrogen production from cellulose in a two-stage process combining fermentation and electrohydrogenesis. International Journal of Hydrogen Energy, 34(15), 6201-6210.
- Logan, B.E., Hamelers, B., Rozendal, R., Schröder, U., Keller, J., Freguia, S., Aelterman, P., Verstraete, W. and Rabaey, K., 2006. Microbial fuel cells: methodology and technology. Environmental science & technology, 40(17), pp.5181-5192.
- Rahimnejad, M., Adhami, A., Darvari, S., Zirepour, A. and Oh, S.E., 2015. Microbial fuel cell as new technology for bioelectricity generation: a review. Alexandria Engineering Journal, 54(3), pp.745-756.
- Sleutels, T. H., Ter Heijne, A., Buisman, C. J., and Hamelers, H. V., 2012. Bioelectrochemical Systems: An Outlook for Practical Applications. ChemSusChem, 5(6), 1012-1019.


